

The configuration of Krypton is very similar to that of neon (Kr8f14d9), which is the most abundant noble gas. Note: Almost all elements do follow Aufbau principle especially elements of lower atomic number.Ģ. The electronic configuration of Krypton is Kr4f13d6, meaning that the ground state has six electrons in the 1s orbital and four in the 2s orbital, which means that it is an s2-type metal. Now, the answer will be- when the 37th electron will go into the 'kr' atom, it will occupy '5s' sublevel as after '4p' ,'5s' this is the next higher energy level.
#Electron configuration krypton full#
The full electron configuration of mercury is 1s2 2s2p6 3s2p6d10 4s2p6d10f14 5s2p6d10 6s2. Where the numbers 1,2,3,4,5 are principal quantum numbers indicated as n and s,p,d,f etc are sublevels under principal quantum number. By Staff Writer Last Updated April 16, 2020. We use the Noble Gas above Silver which is Krypton Kr Ag Kr 5s2 4d9 Remember that the d block orbitals are one level lower then the period they are in on the periodic table. So the increasing order of sub-level based on energy will be-ġs < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d. An electron configuration shows the number and arrangement of the electrons in an atom based upon the period and s,p,d,f. Atomic Number: 36, Atomic Radius: Atomic Symbol: Kr, Melting Point: Atomic Weight: 83.79, Boiling Point: Electron Configuration: Ar4s23d104p Oxidation.

Where Ar is a Noble gas whose atomic number is 18.įrom the electronic configuration, we can understand that all sub-level up to 4p orbitals are fulfilled by thirty six electrons.Īccording to Aufbau rule in the ground state for a multi-electron system electrons first occupy lowest available energy levels completely before occupying higher levels.
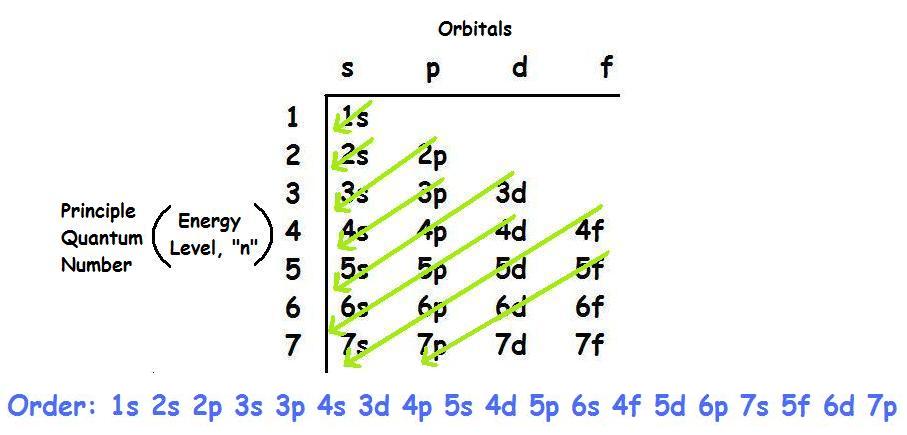
The atomic number of the given atom kr is 36 its electronic configuration is \4\]. According to the Aufbau rule, the 37th electron will go into a higher energy level after '4p' sublevel. Hint: We will explain this question from “Aufbau principle”.


 0 kommentar(er)
0 kommentar(er)
