

During his experiment he discovered electron and it is one of the most important discoveries in the history of physics. Thomson experimenting with cathode ray tubes. Why was the cathode ray experiment important?Ĭathode ray experiment was a result of English physicists named J. Thomson demonstrated that cathode rays could be deflected by a magnetic field, and that their negative charge was not a separate phenomenon. What were 2 major findings or details of JJ Thomson’s experiment? Kaufmann concluded that the hypothesis that cathode rays were particles was inconsistent with this result. Thomson found that cathode rays always had the same e/m ratio, no matter what metals were used for the cathodes and no matter what gas was used in the tubes. What conclusions did JJ Thomson make with his observations of cathode rays? In addition, he also studied positively charged particles in neon gas. He demonstrated that cathode rays were negatively charged. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. What did JJ Thomson discover with the cathode ray?
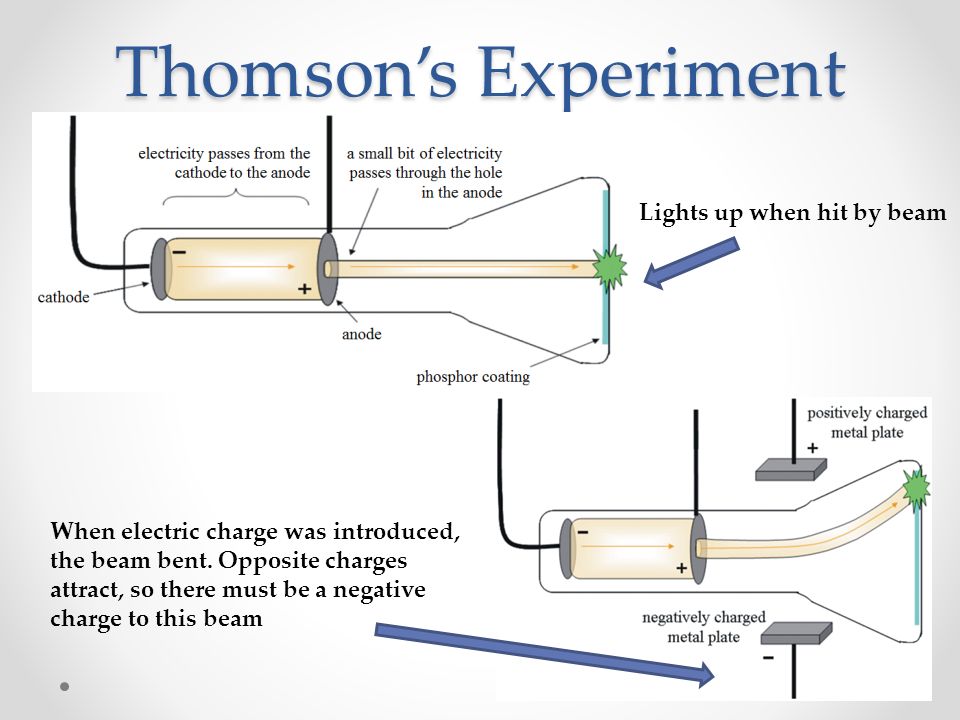
6 Who conducted experiments with cathode ray tubes?.5 What did Thomsons cathode ray tube experiment demonstrate?.3 Why was the cathode ray experiment important?.2 What conclusions did JJ Thomson make with his observations of cathode rays?.1 What did JJ Thomson discover with the cathode ray?.


 0 kommentar(er)
0 kommentar(er)
